Exploring the True Nature of Atoms: A Quantum Perspective
Written on
Chapter 1: Understanding Atoms
What do atoms really look like? Most of us envision them as protons and neutrons held together by electrons that orbit around them. But is this depiction accurate? Over the last century, our comprehension of atoms has evolved significantly, from Rutherford's nuclear experiments to the discovery of neutrons, reshaping our understanding of the universe's fundamental components.
Historically, we were taught that atoms are primarily empty, with a dense core surrounded by tiny electrons in fixed energy levels. However, this interpretation is flawed; electrons are not merely point-like particles. Instead, they exhibit a 'fuzzy' nature, complicating our perception of their behavior.
The concept of the 'Quantum Wave Function' is pivotal here. This term describes the probabilistic nature of an electron's existence rather than its being a singular particle. To illustrate, imagine an isolated electron without nearby atoms or electromagnetic forces. Its 'location' indicates where it is most likely to be found, but the probability of finding it decreases exponentially as you move away from this point. This is the essence of the quantum wave function.
While it is theoretically possible for an electron to vanish from one location and reappear on the other side of the universe, the likelihood is so minuscule that it remains virtually impossible.
We often think of electrons as disappearing and reappearing, leading to the notion of an electron cloud, but this is a simplification. The double-slit experiment provides a clearer insight into the behavior of particles, whether they be photons or electrons.
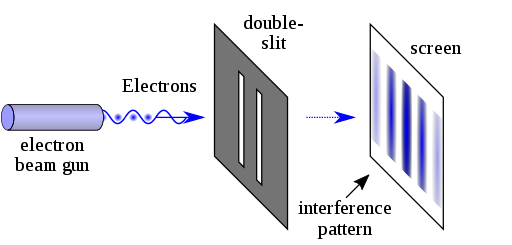
In this experiment, individual photons or electrons are directed through two closely spaced slits, creating an interference pattern on a screen behind the slits. If these particles behaved like point-like objects, we would expect a straightforward bright spot on the screen. Instead, the result is a pattern of light and dark bands, illustrating that the quantum wave function traverses both slits, interfering with itself.

The peculiar nature of quantum physics suggests that as an electron or photon moves, we don't perceive it as a point-like entity; rather, it behaves more like a wave, with probabilities that increase and decrease over distance. This wave-like behavior allows for interference phenomena, producing complex patterns of activity.
Now, let's delve deeper. As electrons or photons pass by, their quantum wave functions act like waves, leading to constructive and destructive interference. This is what generates the interference patterns observed in the double-slit experiment, even when individual particles are used.
The implications for our understanding of atoms are profound. Instead of neatly defined orbits, electrons create a 'fuzzy' cloud around the nucleus, shaped by their quantum wave functions. This electron cloud does not have a fixed distance from the nucleus; rather, it fluctuates, resulting in a hazy distribution of probability.
What about energy levels? In traditional education, we learned that atoms have distinct energy shells where electrons orbit the nucleus. However, if electrons don’t maintain a consistent distance, this model cannot hold true. The reality is that these energy levels correspond to intricate electron clouds that take on diverse and beautiful shapes.
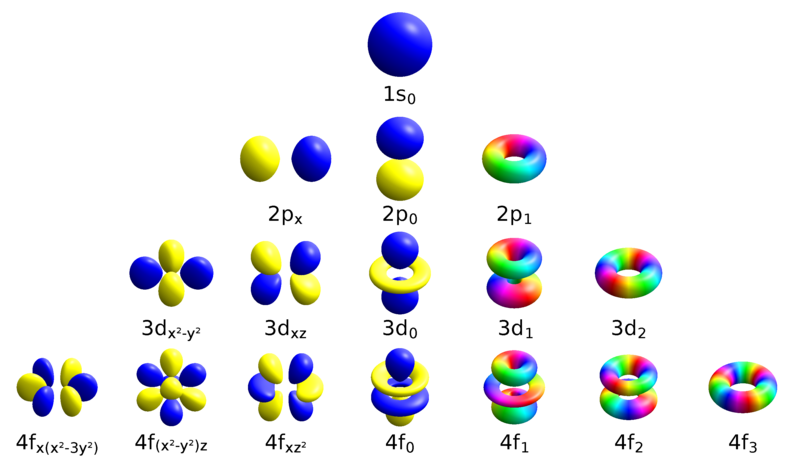
These electron clouds interact with one another, leading to a variety of fascinating formations. The shapes of these clouds can shift with different energy levels, illustrating how a simple atom like hydrogen can present multiple electron cloud patterns.
These unconventional shapes significantly influence chemical bonding. Each unique electron cloud provides points of connectivity for elements to bond, leading to various structural possibilities.
Chapter 2: Visualizing Atoms
So, if we could capture an image of an atom, what would it reveal? The answer lies in fuzzy, opaque shapes that exhibit a kaleidoscopic quality. These atoms would be surrounded by intricate electron clouds, some resembling spheres while others take on more complex forms. As atoms bond, they share these clouds, resembling entities holding hands.
Ultimately, atoms are not merely empty space; they are filled with dynamic electron clouds. This understanding transforms our perspective on atomic structure, showcasing a stunningly beautiful atomic world filled with complex, symmetrical forms that appear almost floral in nature.
The next time someone asserts that atoms are predominantly voids, you can enlighten them by explaining that they are, in fact, brimming with intricate electron clouds, revealing the remarkable beauty inherent in the universe's smallest components.
The first video titled "What Does An Atom REALLY Look Like?" offers an engaging exploration of atomic structures and challenges conventional views, delving into the complexities of electron behavior.
The second video, "Not All Your Atoms are Stardust," further expands on the fascinating and unexpected realities of atomic composition, inviting viewers to reconsider their understanding of the universe's building blocks.