Unraveling COVID-19 Immunity: Insights from Antibody Studies
Written on
Chapter 1: Understanding Antibody Responses
Recent findings suggest that certain individuals possess IgG antibodies capable of recognizing the coronavirus responsible for COVID-19. The study of antibody responses to SARS-CoV-2 has emerged as a critical element in understanding the severity of COVID-19 symptoms. Initial investigations into how the body generates antibodies against this virus have begun to surface. Although some of these findings are preliminary and pending peer review, they shed light on the variability of immune responses to SARS-CoV-2.
Gaining insights into the antibody-mediated response is essential for determining the potential for immunity following exposure, the duration of such immunity, and the methods for testing it. Furthermore, differences in these antibody responses may influence the severity of the disease through a mechanism known as antibody-dependent enhancement (ADE).
Unexpectedly Rapid Development of IgG Antibodies
Suthar and colleagues focused their research on the immune response to the Spike (S) protein of the virus, particularly the receptor-binding domain (RBD) that interacts with human cell receptors. The S protein, which lends coronaviruses their characteristic crown-like appearance in visual representations, is crucial for the virus's ability to infect host cells.
While all coronaviruses possess an S protein, the specific amino acid sequences vary among different strains. The closest relative to SARS-CoV-2 is SARS-CoV, which shares only about 73% similarity in its S protein structure. Additionally, the S proteins undergo glycosylation, a modification process involving sugar attachments by enzymes in infected cells, leading to further variability in immune responses. This variability explains why infection with one coronavirus does not always lead to immunity against another.
Investigating antibodies that target the S protein is vital, as these antibodies can block the virus from entering human cells by interfering with the interaction between the S protein and ACE2, a key receptor on human cells. Antibodies that successfully inhibit this interaction are termed “neutralizing antibodies.”
A key focus of Suthar’s study was the IgG antibody, one of five types of antibodies differentiated by their heavy chains. IgM antibodies are typically the first to appear during an initial infection, while IgG antibodies are produced upon re-exposure to the pathogen.
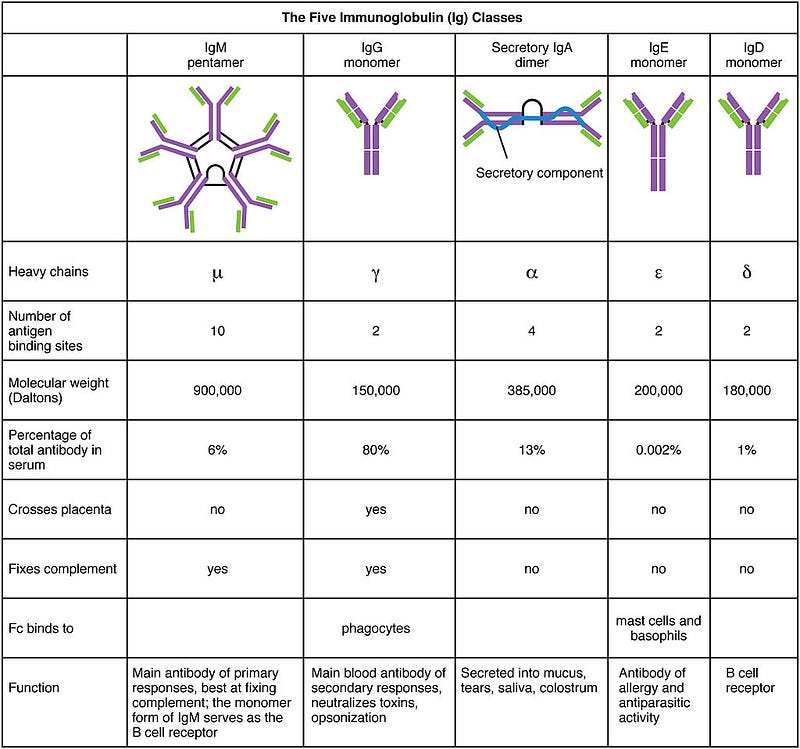
By measuring IgG antibodies that recognize the RBD, the researchers aimed to identify antibodies most likely to provide neutralization and immunity.
The research team initially assessed RBD antibodies in 44 COVID-19 patients, comparing their samples to blood plasma from 12 healthy individuals collected prior to the emergence of SARS-CoV-2. They generated and purified the RBD from the S protein for testing, which included amino acids 319 to 541. The assay used could detect the presence and type of antibodies in the serum samples.
Among the 44 infected individuals, 36 exhibited detectable RBD-specific IgG antibodies, though the amounts varied significantly. Antibody levels are quantified using a “titer,” indicating how much a sample can be diluted before antibodies become undetectable. In this study, IgG RBD antibody titers ranged from below 100 to 142,765, showing a wide range of responses among patients.
Some patients, particularly those with very low or undetectable IgG titers, had higher IgM levels, indicating a complex immune response. The balance between IgM and IgG can reveal the timing of infection and whether a response is from an initial or subsequent infection.
How could some patients present high IgG levels while having low IgM? There are several possibilities:
- Patients may be in the later stages of their first infection, with B cells transitioning from IgM to IgG production.
- Some individuals may have had prior exposures to SARS-CoV-2, leading to a secondary immune response.
- Previous infections with other coronaviruses might trigger cross-reactive IgG responses.
The study noted variability in symptom duration among participants, which may affect the timing of antibody responses.
Antibodies That Neutralize SARS-CoV-2
Another critical aspect of Suthar's research involved testing whether patients' antibodies could neutralize the virus. This was done by mixing diluted plasma samples with SARS-CoV-2 and observing the effects on cultured cells. Out of the 44 patients, 40 showed evidence of neutralizing antibodies.
The neutralizing capacities of these antibodies varied widely, with some being effective even when diluted up to 5,763 times, while others were only effective at lower dilutions. Not all antibodies against a virus are neutralizing; some may target viral proteins that become exposed as the immune system attacks infected cells, while others may bind but not prevent viral entry.
Predicting Neutralizing Antibodies from RBD-Specific IgG Antibody Titer
To ascertain if RBD-specific IgG antibodies could reliably predict neutralizing activity, researchers analyzed a larger sample set of 231 confirmed COVID-19 cases within 22 days of positive SARS-CoV-2 testing. They categorized the samples based on the time post-positive testing.
Their analysis revealed that the presence of RBD-specific antibodies was a strong indicator of neutralizing activity, suggesting a reliable method for assessing potential immunity to SARS-CoV-2.
This study raises questions about whether some hospitalized patients are experiencing their first infection or responding to previous exposures. It also highlights the importance of timing in testing for neutralizing antibodies.
Importantly, the research did not determine if the detected antibodies confer protection against reinfection or lessen disease severity. Notably, not all patients exhibited detectable antibodies; eight out of the initial 44 tested negative for RBD-specific antibodies, suggesting that they either tested too early or produced antibodies that did not recognize the S protein.
The study referenced is a preprint and has not undergone peer review.
Highlighted Article
Also of Interest
Interferon Responses Could Explain Susceptibility to Severe COVID-19
Impaired or delayed antiviral signaling could be a treatable cause of serious COVID-19.
The first video titled "Do some people have 'super immunity' to Covid?" discusses the phenomenon of heightened immune responses in certain individuals, exploring the concept of 'super immunity' and its implications for understanding COVID-19.
The second video "Antibody responses to SARS-CoV-2 infection and vaccination - Florian Krammer Ph.D." presents expert insights on the immune response to COVID-19, covering antibody production in relation to infection and vaccination.