# The Fascinating Science Behind Christmas Crackers and Their 'Crack'
Written on
Chapter 1: The Tradition of Christmas Crackers
Do Christmas crackers feature in your holiday celebrations? For many in the UK, the answer is undoubtedly yes! The concept of the Christmas cracker is credited to confectioner Tom Smith, who introduced it in 1861. He was inspired by the beautifully wrapped sugared almonds he saw in Paris and originally named them ‘Bangs of Expectation’—a truly poetic touch.
As we gather for our eagerly awaited festive feast, we are always welcomed by a colorful array of these paper tubes, sparkling in the gentle glow of candlelight. Before diving into the meal, we turn to our neighbors, offering one end of these 'Bangs of Expectation' while exchanging knowing glances, subtly flexing our arms beneath our cozy Christmas sweaters.
We count together: “One, two, THREE!”
On “THREE!” we both pull apart our cracker, tearing through the shiny wrapping with gusto. In that moment, a loud ‘crack’ resonates, accompanied by a whiff of gunpowder. Traditionally, at least in my household, the person who manages to pull the larger portion of the cracker during this mini-explosion claims its contents: typically a paper hat, a joke, and some quirky plastic trinket.

Photo by Ian van der Linde on Unsplash
Despite my best efforts, I often find myself with the shorter end of the cracker, regardless of how many bicep curls I complete. Thankfully, someone usually shows me some holiday kindness and shares their winnings—a little goodwill that captures the spirit of the season.
Section 1.1: The Chemistry of Christmas Crackers
Looking for a way to entertain your family and friends beyond the usual cringe-worthy Christmas cracker jokes? Why not share some festive facts about the chemistry behind these delightful items?
Christmas crackers contain a small quantity of an explosive substance, such as gunpowder (a mix of potassium nitrate, charcoal, and sulfur) or silver fulminate. This compound, a salt derived from fulminic acid, is highly unstable and can even explode under its own weight if too much is present.
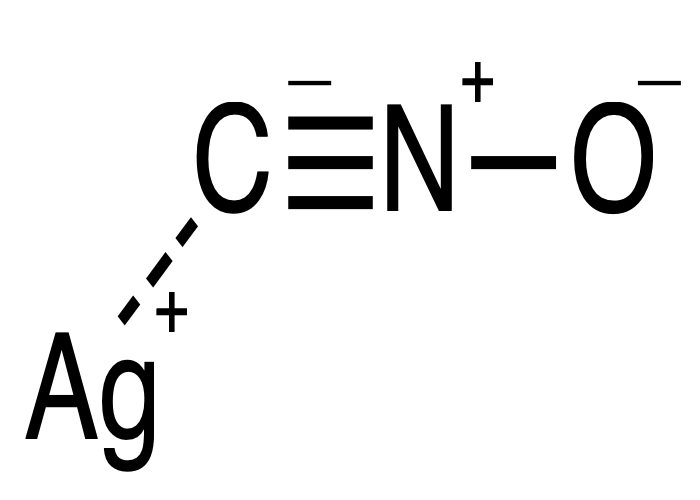
Fred the Oysteri. The source code of this SVG is valid. This vector image was created with Adobe Illustrator. CC0, via Wikimedia Commons.
This remarkable sensitivity limits the applications of silver fulminate, but manufacturers have cleverly harnessed this friction explosive for novelty items like Christmas crackers. Each cracker comprises two pieces of card: one coated with a small amount of explosive and the other featuring a rough texture. When the cracker is pulled, the resulting friction triggers the explosion. The cylindrical design of the cracker enhances the sound for dramatic effect.
If silver fulminate is used, the nitrogen-oxygen bond breaks as the nitrogen atom preferentially combines with nitrogen in the air. Of course, since we’re dealing with explosives, it’s crucial to adhere to the instructions provided.
Section 1.2: The Joy of Chemistry at the Dinner Table
Rest assured, your guests will enjoy explosive entertainment whether you choose to retell your hilariously bad jokes this year—all thanks to a touch of festive chemistry!
Key sources: ‘Why do Christmas crackers go bang?’, The Open University, 2010. ‘Christmas Cracker Chemistry’, Compound Interest, December 2013.
Chapter 2: Learn More About the Author
Want to delve deeper into my stories and background? Check out the link below:
Who I am and what I like to write about (plus a disclaimer for my stories — yawn!)
Ah, I see you have stumbled upon my profile page — lovely to meet you!